US FDA Recalls Systane Eye Drops For Possible Fungal Contamination
Dec 26, 2024
News
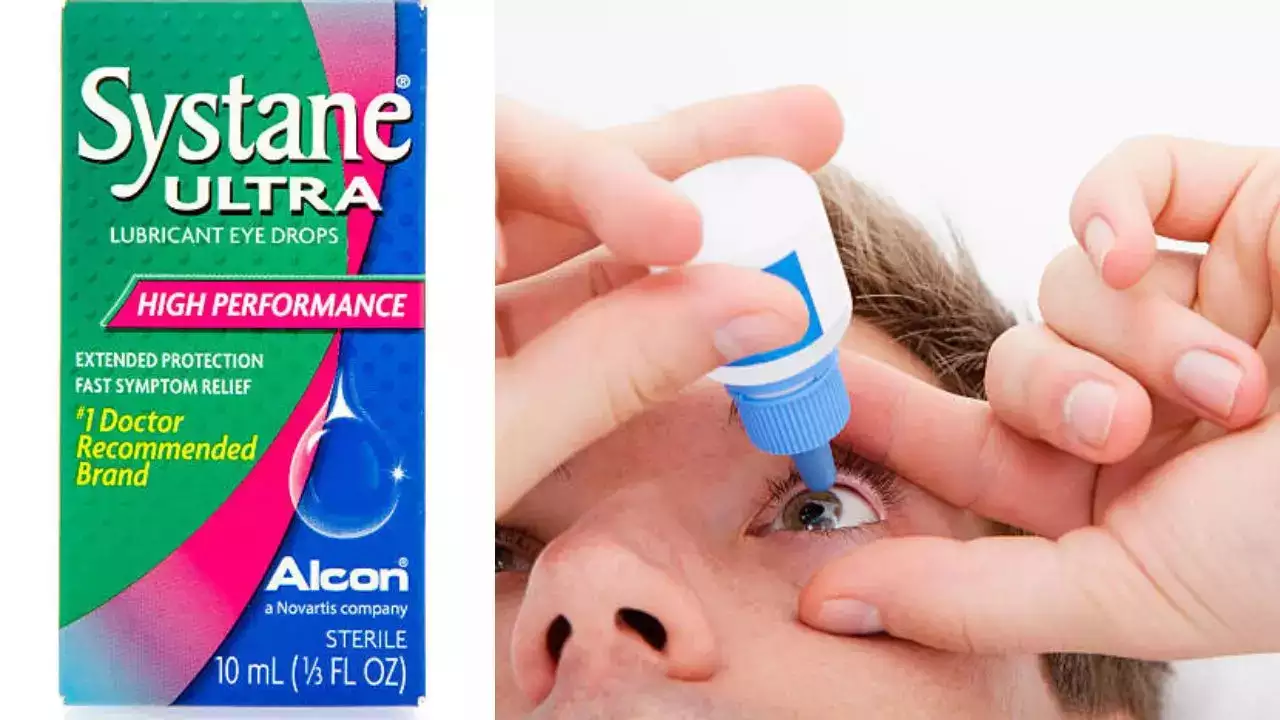
The contamination could lead to severe eye infections that can even make you blind
Systane brand eye drops were voluntarily recalled due to possible fungal contamination, the United States Food and Drug Administration said. A single lot of Systane Lubricant Eye Drops Ultra PF was recalled following a consumer complaint "of foreign material observed inside a sealed single-use vial," the FDA said in a press release.
According to the release, the material was “fungal in nature”.
Experts say the contamination could lead to severe eye infections that can even make you blind and in very rare cases even be potentially life-threatening in immunocompromised patients.
No adverse customer reports received
According to Alcon Laboratories - manufacturers of the eye drops – there have been no reports received of any customers suffering adverse effects till now, The recalled eye drops come in 25-count on-the-go single vials with lot number 10101 and an expiration date of September 2025. The product was sold at major grocery chains across the US.
Steven Smith, an Alcon spokesperson, said that the company's investigation is ongoing, but "the presence of foreign material appears to be isolated to the single unit returned by a customer." Alcon recalled the product "out of an abundance of caution to prioritize consumer safety," he added.
What are Systane eye drops used for?
Systane eye drops are mostly used to relieve dry and irritated eyes. They work by hydrating and lubricating the eye's surface, which helps with symptoms like burning, itching, and a feeling of something in the eye.
They also help relieve burning and other symptoms of dry eyes. Doctors say the product contains one or more of the following ingredients:
- Carboxymethylcellulose
- Dextran
- Glycerin
- Hypromellose
- Polyethylene glycol 400
- Polysorbate
- Polyvinyl alcohol
- Povidone
According to experts, while you must follow all directions on the product package, use it only when it has been prescribed by your doctor.
Use this medication in the affected eyes as directed.
Do not use a solution that has changed colour or is cloudy. Certain brands, which contain glycerin with polysorbates, among other ingredients, may have a milky appearance. This is okay as long as the solution does not change colour. Some eye drops need to be shaken before use. Check the label to see if you should shake your product before using it.
Get Latest News Live on Times Now along with Breaking News and Top Headlines from Health and around the world.



